Research
Project 1: Physical model of activation of voltage-sensitive ion channels
Co-workers: Dr. Cliff Chancey, Purdue University - Calumet, Hammond, IN;
Amherst College students Peter Marshall '91, Haitao Huang '94, Colin Stewart '94, and
Damien Sheehan-Connor '95.
Voltage-sensitive ion channels fluctuate between open and closed states on a millisecond time scale. Statistical characteristics of these fluctuations, such as the fraction of time spent in the open state and the distributions of open and closed times, are functions of membrane potential and temperature. We are attempting to account for the behavior of sodium channels by evaluating forces on the S4 alpha-helix portion of the channel molecule, using the "sliding hel
ix" concept of activation. This model is illustrated in the accompanying figure, showing the position of charged residues on the S4 helix and on other transmembrane segments near the S4 helix.
 Positive charges in the helix are shown by
open circles, and putative negative charges on adjacent segments of the channel molecule by filled circles. During activation, the helix is assumed to move rigidly outward, with no changes in the distances between positively charged residues within the helix, and no changes in the positions of the negative charges.
Positive charges in the helix are shown by
open circles, and putative negative charges on adjacent segments of the channel molecule by filled circles. During activation, the helix is assumed to move rigidly outward, with no changes in the distances between positively charged residues within the helix, and no changes in the positions of the negative charges.
The interaction between the S4 helix segment and its environment was modelled by
- nearest neighbor coulombic forces
- the electric force due to an external electric field, and
- static mechanical and electrostatic forces.
These terms collectively describe a depolarization-dependent effective potential within which the S4 segment moves.
Our analysis makes several simplifying assumptions:
- that all four S4 segments are identical, with each containing six positively charged arginine or lysine residues;
- that they operate independently;
- that the conformational change of the S4 segment is accomplished by a sliding translation which follows a left-handed helical path;
- that the channel pore is open if and only if at least three of the four homologous repeats in the sodium channel molecule are activated;
- that activation may be modelled independently of inactivation; and
- that Batrachotoxin-treated axons, although different in many respects from native axons, possess normal gating mechanisms, so that data from Batrachotoxin-treated axons may be used for comparison with the results from the model.
Thermal transitions between center-of-mass energy states of the segment were modelled starting from the Boltzmann distribution, and the time evolution of the segment's position relative to the membrane was simulated using a computer program.
Combining the histories of four such processes models the activation history of the channel molecule.
 This figure shows (1) the double-well potential at the left; (2) colored dots representing the state of the S4 segment at several hundred successive times, with green=non-activated, red=activated, and white=fluctuating; and (3) the channel opening and closing, represented as a "patch record" at bottom, calculated by combining four similar calculations representing each of four homologous repeats in the channel.
This figure shows (1) the double-well potential at the left; (2) colored dots representing the state of the S4 segment at several hundred successive times, with green=non-activated, red=activated, and white=fluctuating; and (3) the channel opening and closing, represented as a "patch record" at bottom, calculated by combining four similar calculations representing each of four homologous repeats in the channel.
The results of the model simulations agree well with Batrachotoxin-modified single channel open and closed dwell time distributions (Fig. 3) and with such channels' open probability as functions of depolarization. A and C represent the distribution of closed times over 4 log units of time; B and D represent the distribution of open times. The upper histograms (A and B) are from the model; the lower pair (C and D) are from data of Correa et al. The model also qualitatively agrees
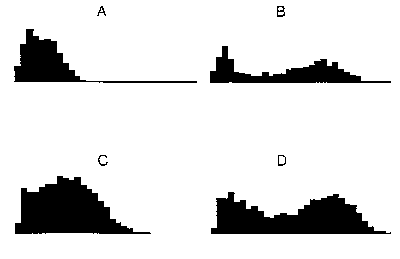 with site-specific mutagenesis experiments which show the different effects of eliminating positive charges on the cytoplasmic and extracellular ends of the S4 segment. However, the temperature dependence of channel opening as predicted by our model is considerably less than he actual temperature dependence of batrachotoxin-modified channels.
with site-specific mutagenesis experiments which show the different effects of eliminating positive charges on the cytoplasmic and extracellular ends of the S4 segment. However, the temperature dependence of channel opening as predicted by our model is considerably less than he actual temperature dependence of batrachotoxin-modified channels.
We have also used the model to investigate the possibility of quantum tunneling of the S4 helix between non-activated and activated states, and found that, when the helix occupies an energy level near the top of either the activated or non-activated potential wells, tunneling is predicted to occur in times of the order of milliseconds, which corresponds to the actual transition times for the channel. Therefore we believe that tunneling is a plausible mechanism of activation transitions, along with classical thermal transitions.
REFERENCES
Chancey, C.C. and S.A. George. Physical model of voltage sensing in sodium channels based on the sliding helix concept. Physical Review E 53: 5137-5145 (1996).
Chancey, C.C., S.A. George, and P.J. Marshall. Calculations of quantum tunnelling
between closed and open states of sodium channels. J. Biological Physics 18:307-321 (1992).
Chancey, C.C., S. A. George, and H. Huang. Dynamical model of sodium channel
activation. Biophys. J. 66: A103 (1994)
Conti, F. and W. Stuhmer. Quantal charge redistributions accompanying the
structural transitions of sodium channels. Euro. Biophys. J. 17:53-59 (1989)
Correa, A.M., F. Bezanilla, and R. Latorre. Gating kinetics of Batrachotoxin-
modified Na+ channels in the squid giant axon. Biophys. J. 61:1332-1352 (1992).
Guy, H.R., and P. Seetharamulu. Molecular model of the action potential sodium
channel. Proc. Natl. Acad. Sci. USA. 83:508-512 (1986).
 Positive charges in the helix are shown by
open circles, and putative negative charges on adjacent segments of the channel molecule by filled circles. During activation, the helix is assumed to move rigidly outward, with no changes in the distances between positively charged residues within the helix, and no changes in the positions of the negative charges.
Positive charges in the helix are shown by
open circles, and putative negative charges on adjacent segments of the channel molecule by filled circles. During activation, the helix is assumed to move rigidly outward, with no changes in the distances between positively charged residues within the helix, and no changes in the positions of the negative charges.
 This figure shows (1) the double-well potential at the left; (2) colored dots representing the state of the S4 segment at several hundred successive times, with green=non-activated, red=activated, and white=fluctuating; and (3) the channel opening and closing, represented as a "patch record" at bottom, calculated by combining four similar calculations representing each of four homologous repeats in the channel.
This figure shows (1) the double-well potential at the left; (2) colored dots representing the state of the S4 segment at several hundred successive times, with green=non-activated, red=activated, and white=fluctuating; and (3) the channel opening and closing, represented as a "patch record" at bottom, calculated by combining four similar calculations representing each of four homologous repeats in the channel.  with site-specific mutagenesis experiments which show the different effects of eliminating positive charges on the cytoplasmic and extracellular ends of the S4 segment. However, the temperature dependence of channel opening as predicted by our model is considerably less than he actual temperature dependence of batrachotoxin-modified channels.
with site-specific mutagenesis experiments which show the different effects of eliminating positive charges on the cytoplasmic and extracellular ends of the S4 segment. However, the temperature dependence of channel opening as predicted by our model is considerably less than he actual temperature dependence of batrachotoxin-modified channels.